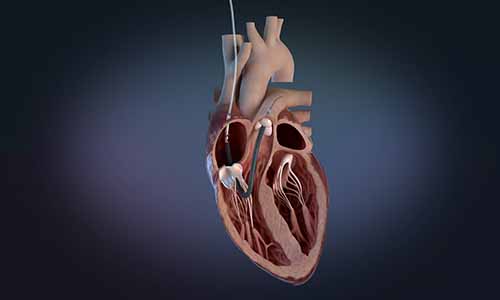
DANVERS : Abiomed announces the first three patients in the world have been treated with Impella RP Flex with SmartAssist, Abiomed’s newest heart pump for patients experiencing right heart failure. All three patients have now been successfully weaned off Impella support and two have already returned home with their native heart. The patients were treated at Hackensack University Medical Center/Hackensack Meridian Health in Hackensack, N.J., and Kingwood Medical Center in Kingwood, Texas.
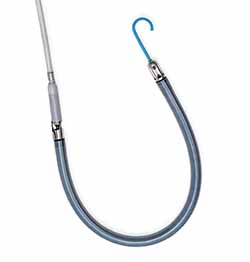
Impella RP Flex is implanted percutaneously through the internal jugular (IJ) vein, which provides the option for patient mobility while on support, and is designed to be easy to implant, with a flexible canula that is advanced over an extra-supportive guidewire. It includes SmartAssist dual-sensor technology with Impella Connect, giving health care providers the ability to monitor the pump remotely from any internet-connected device and providing advanced metrics to help with pump management and weaning. It is also compatible with a sodium bicarbonate purge solution to simplify patient anticoagulation management.
“Impella RP Flex is an innovative technology that can allow right heart failure patients to be mobile while on support,” said Mark Anderson, MD, chairman of the department of cardiac surgery and a cardiothoracic surgeon at the Heart and Vascular Hospital at Hackensack University Medical Center/Hackensack Meridian Health, who led the medical team for the world’s first Impella RP Flex implant.
Dr. Anderson and his colleague Yuriy Dudiy, MD, implanted Impella RP Flex on a 71-year-old patient who was having a minimally invasive valve surgery. The patient received Impella RP Flex support for five days while their heart rested and recovered. Dr. Anderson’s second Impella RP Flex case involved a 51-year-old patient who experienced cardiac arrest and received Impella support for four days after valve surgery.
”Right ventricular failure is important to identify and treat early, and Impella RP Flex will enable more patients to achieve native heart recovery,” said Robert Salazar, MD, an interventional cardiologist and director of cardiovascular research at Kingwood Medical Center. Dr. Salazar and his colleague Marloe Prince, MD, implanted Impella RP Flex in a 75-year-old patient following a thrombectomy procedure for a pulmonary embolism. The patient remained on support for four days and is expected to be discharged from the hospital in the coming days.
Impella RP Flex is the latest iteration of the Impella RP heart pump. The U.S. Food and Drug Administration (FDA) granted Impella RP approval under a humanitarian device exemption (HDE) in 2015, followed by the pre-market approval (PMA), its highest level of approval as safe and effective in 2017. In October 2022, the FDA granted Impella RP Flex a PMA to treat acute right heart failure for up to 14 days and the first patients were treated in November 2022.
Also in October 2022, the FDA accepted and closed the Impella RP post-approval study, which enrolled 110 patients at 29 study sites. The study represented real world experience and identified the best practice of treating right heart failure early. Data published in the Journal of Heart and Lung Transplantation shows patients who received Impella RP support within 48 hours of cardiogenic shock onset had a significantly higher survival rate than those who received delayed right-heart support (72% vs. 14%, p<0.001, Anderson et al.).
Impella RP Flex is being introduced in the U.S. through a controlled rollout at leading centers for heart recovery.